Technology
An innovative natural enzyme

The problem
A protein hard to digest
Wheat is a major staple cereal grain consumed worldwide that provides a major source of high-quality nutrition to humankind. However, for a small subset of the population, a range of wheat components, principally proteins, are associated with several important medical illnesses that can affect patient health and quality of life and, in some cases, can be life-threatening.
Gluten comprises of a group of prolamins found in wheat, rye, barley. When consumed, gluten resists complete digestion by human intestinal digestive endo-proteases, pepsin, trypsin, chymotrypsin, and carboxypeptidase primarily due to the high content of proline residues*.
Undegraded immuno-reactive peptides, such as the 33-mer α2-gliadin peptide, the 26-mer γ5-gliadin peptide and the α-gliadin 31-43 (p31-43)* interact with the intestinal lining, initiating the cascade of effects that characterise the conditions in the wide spectrum of gluten related disorders.
A second protein found in most gluten-containing grains, α-amylase trypsin inhibitors (ATI), is also resistant to intestinal enzymatic digestion and has recently discovered as one of the main triggers in IgE-negative wheat allergy and potentially non-coeliac wheat (‘gluten’) sensitivity*. Moreover, ATIs have been shown to promote inflammation of the central nervous system, potentially playing a role in human multiple sclerosis*.
The only recognised treatment for gluten related disorders is a lifelong adherence to a strict gluten free diet; which implies that all gluten-containing foods and meals, produced from wheat, rye, barley, and must be completely excluded from the diet. Nevertheless, such a diet is difficult to follow due to unintended contamination of “gluten-free” products, improper labelling, social constraints, and ubiquity of gluten in foods and pharmaceuticals. Thus, accidental gluten encounters are common and ongoing consumption of “background” gluten is believed to account for ongoing symptoms, inflammation* and, in the case of coeliac disease, persistent intestinal damage*.

The impact on quality of life
of people with coeliac disease had identified ongoing intestinal damage, likely due to regular accidental gluten ingestion*
of people with non-coeliac gluten sensitivity reported accidental consumption of gluten at least once in four weeks despite maintaining a gluten free diet*
of people with coeliac disease report inadvertent gluten consumption on a regular basis despite trying to maintain a gluten free diet*
Avoiding gluten contamination and the resulting symptoms often results in reduced social interactions and travel; increased hypervigilance, self-isolation, fatigue, and anxiety, leading to a reduced emotional quality of life*
The solution
Clinically proven gluten digestive support
Led by Professor Hugh Cornell, the Glutagen team hypothesised that if the right enzyme is supplemented to the gastrointestinal tract prior to gluten consumption it may be capable of preventing the cascade of events observed in coeliac disease and other forms of gluten sensitivities.
The Glutagen team identified that a unique enzyme, caricain, from the skin of the unripe Carica Papaya fruit, is capable of breaking down gluten’s toxic and immunogenic peptides. Moreover, recent studies showed that caricain is also capable of breaking down ATI’s in-vitro.*
The combination of caricain’s high activity, unique specificity and the use of an enteric coating was shown to be effective at protecting patients suffering from coeliac disease and dermatitis herpetiformis from a daily gluten challenge. A gluten challenge that resembled the ongoing ingestion of background gluten.
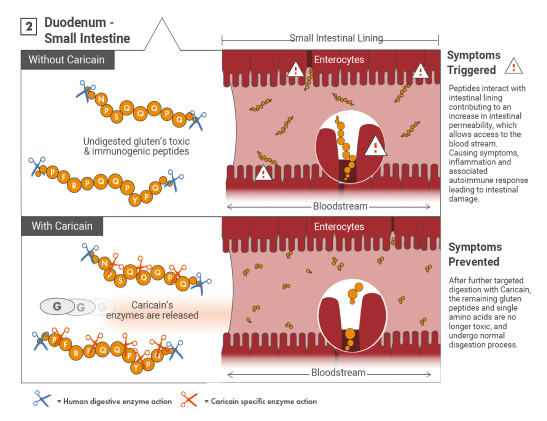
How does it work?


GluteGuard
Enhancing gluten free diet compliance for the community
GluteGuard, an enterically coated tablet containing Caricain, is the world’s only clinically proven protection from the effects of accidental gluten consumption. Taken before a meal, GluteGuard helps by providing support in maintaining a gluten free diet. Suitable for anyone who is required to follow a gluten free diet for medical reasons including those with coeliac disease, non-coeliac gluten sensitivity, dermatitis herpetiformis and other gluten related disorders.
Not a replacement for the gluten free diet, but an adjunct to it, GluteGuard is to be used in situations where sufferers cannot be certain that a gluten free meal is without gluten-contamination. Particularly in situations such as:
 Cultural or language barriers can make avoiding gluten ingestion significantly challenging when consuming meals prepared by others.
Cultural or language barriers can make avoiding gluten ingestion significantly challenging when consuming meals prepared by others.
 Events catered by others near gluten-containing foods significantly raise the risk of cross-contamination.
Events catered by others near gluten-containing foods significantly raise the risk of cross-contamination.
 Relying on staff’s knowledge of gluten free ingredients and safe practices is necessary even when gluten free options are available.
Relying on staff’s knowledge of gluten free ingredients and safe practices is necessary even when gluten free options are available.
 When product labelling is unclear and there is difficulty ensuring specific ingredients are gluten free.
When product labelling is unclear and there is difficulty ensuring specific ingredients are gluten free.
The results? Life changing.
Ella H, Victoria, Australia
“[GluteGuard] has been the most incredible product! It has allowed me the freedom of not worrying about cross-contamination at restaurants, and freedom I didn’t think I would ever have when travelling overseas.”
Amber S, New South Wales, Australia
“This product has changed my life! No longer are the crippling stomach aches followed by any meal, or the general sluggish feeling. Three weeks on GluteGuard and I haven’t looked back. Thank you so much!”
Ariane S, Victoria, Australia
“I can now confidently eat gluten free foods in restaurants and also when travelling internationally. Literally life changing to stop living in fear of dining out.”
With Glutagen, you stay ahead of the curve in gluten science.
Research and development
Clinical trials
Publications
Investigated gliadin-detoxifying enzymes in porcine intestinal extract and papaya latex using the rat liver lysosomal assay.
Investigated the protective effect of caricain using the rat liver lysosomal assay and explored enzyme kinetics.
Explored the detoxifying effect of caricain in baked goods and studied gliadin residues using enzyme-linked immunosorbent assay test kit (ELISA).
Randomized placebo controlled clinical trial exploring the protective effect of caricain in dermatitis herpetiformis patients.
Investigated the relative rate of disappearance of coeliac relevant gluten epitopes in extracts treated with eight commercial supplements and caricain using two competitive enzyme-linked immunosorbent assays.
Randomized placebo controlled clinical trial exploring the protective effect of caricain in coeliac disease patients.
Randomized placebo controlled clinical trial exploring the protective effect of caricain in coeliac disease patients.

